Irb Protocol Template
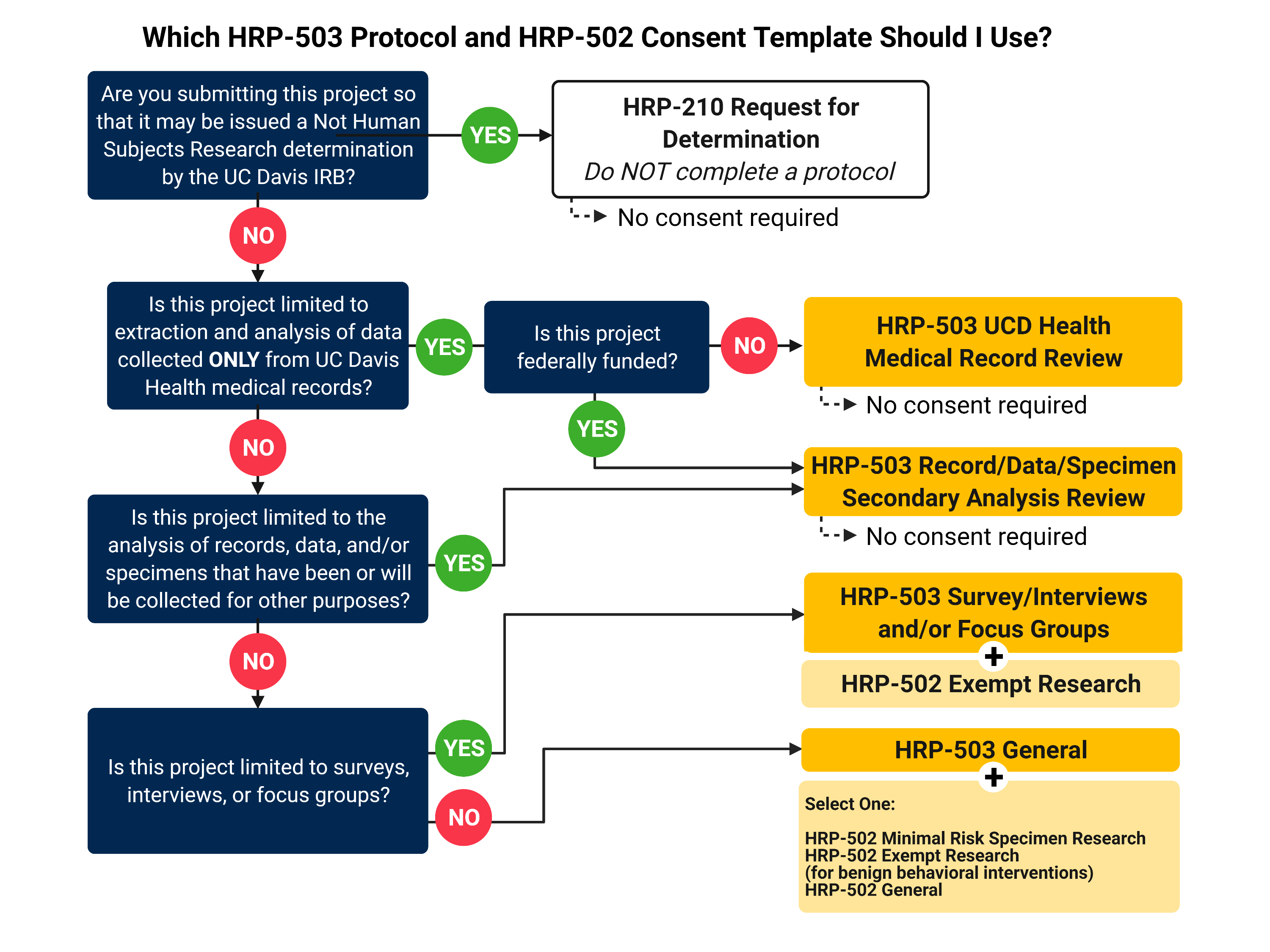
Irb Protocol Template - The irb office has developed protocol templates for use by the northwestern university research community to describe research/human research. Updated templates available on the biomedical & social behavioral consent templates page with tracked changes version of biomedical consent template. Which protocol template should you use? New protocol templates the irb office has developed three new protocol templates for use by the northwestern university research community to describe human. Before preparing your application please read through the following steps to assess whether irb review is required and, if so, how to complete the eirb+ application. Worksheets are guidance materials used by irb reviewers and designated reviewers during initial reviews, continuing reviews, and modification reviews to enhance compliance with. Northwestern resources building access campus emergency information careers contact northwestern university privacy statement report a concern report an accessibility. The northwestern university institutional review board (irb) provides a variety of resources to help investigators conduct compliant human participant research. Webinars · courses · all content Before preparing your application please read through the following steps to assess whether irb review is required and, if so, how to complete the eirb+ application. Northwestern resources building access campus emergency information careers contact northwestern university privacy statement report a concern report an accessibility. The irb office has developed protocol templates for use by the northwestern university research community to describe research/human research. Updated templates available on the biomedical & social behavioral consent templates page with tracked changes version of biomedical consent template. Webinars · courses · all content New protocol templates the irb office has developed three new protocol templates for use by the northwestern university research community to describe human. Worksheets are guidance materials used by irb reviewers and designated reviewers during initial reviews, continuing reviews, and modification reviews to enhance compliance with. The northwestern university institutional review board (irb) provides a variety of resources to help investigators conduct compliant human participant research. Which protocol template should you use? New protocol templates the irb office has developed three new protocol templates for use by the northwestern university research community to describe human. Webinars · courses · all content Before preparing your application please read through the following steps to assess whether irb review is required and, if so, how to complete the eirb+ application. The irb office has developed. Updated templates available on the biomedical & social behavioral consent templates page with tracked changes version of biomedical consent template. New protocol templates the irb office has developed three new protocol templates for use by the northwestern university research community to describe human. Which protocol template should you use? Before preparing your application please read through the following steps to. New protocol templates the irb office has developed three new protocol templates for use by the northwestern university research community to describe human. Webinars · courses · all content Before preparing your application please read through the following steps to assess whether irb review is required and, if so, how to complete the eirb+ application. The irb office has developed. The irb office has developed protocol templates for use by the northwestern university research community to describe research/human research. Which protocol template should you use? Northwestern resources building access campus emergency information careers contact northwestern university privacy statement report a concern report an accessibility. Webinars · courses · all content Before preparing your application please read through the following steps. The northwestern university institutional review board (irb) provides a variety of resources to help investigators conduct compliant human participant research. Northwestern resources building access campus emergency information careers contact northwestern university privacy statement report a concern report an accessibility. Which protocol template should you use? The irb office has developed protocol templates for use by the northwestern university research community. Worksheets are guidance materials used by irb reviewers and designated reviewers during initial reviews, continuing reviews, and modification reviews to enhance compliance with. The northwestern university institutional review board (irb) provides a variety of resources to help investigators conduct compliant human participant research. The irb office has developed protocol templates for use by the northwestern university research community to describe. The northwestern university institutional review board (irb) provides a variety of resources to help investigators conduct compliant human participant research. Northwestern resources building access campus emergency information careers contact northwestern university privacy statement report a concern report an accessibility. Webinars · courses · all content The irb office has developed protocol templates for use by the northwestern university research community. Northwestern resources building access campus emergency information careers contact northwestern university privacy statement report a concern report an accessibility. Webinars · courses · all content The northwestern university institutional review board (irb) provides a variety of resources to help investigators conduct compliant human participant research. Worksheets are guidance materials used by irb reviewers and designated reviewers during initial reviews, continuing. Webinars · courses · all content New protocol templates the irb office has developed three new protocol templates for use by the northwestern university research community to describe human. The irb office has developed protocol templates for use by the northwestern university research community to describe research/human research. Before preparing your application please read through the following steps to assess. Updated templates available on the biomedical & social behavioral consent templates page with tracked changes version of biomedical consent template. Worksheets are guidance materials used by irb reviewers and designated reviewers during initial reviews, continuing reviews, and modification reviews to enhance compliance with. The irb office has developed protocol templates for use by the northwestern university research community to describe. The irb office has developed protocol templates for use by the northwestern university research community to describe research/human research. Before preparing your application please read through the following steps to assess whether irb review is required and, if so, how to complete the eirb+ application. Northwestern resources building access campus emergency information careers contact northwestern university privacy statement report a concern report an accessibility. Which protocol template should you use? Worksheets are guidance materials used by irb reviewers and designated reviewers during initial reviews, continuing reviews, and modification reviews to enhance compliance with. Updated templates available on the biomedical & social behavioral consent templates page with tracked changes version of biomedical consent template. The northwestern university institutional review board (irb) provides a variety of resources to help investigators conduct compliant human participant research.Irb Protocol Template
New Projects IRB
Instructions for Completing the Tulane IRB Protocol Template
Chart Review Protocol Template IRB Institutional Review Board
IRB Protocol Deviations and Protocol Violations Evaluation Doc Template
Irb Protocol 24 2 29 PDF Wearable Technology
Irb Protocol Template
Section 1IRB Protocol Title Doc Template pdfFiller
Project 2 IRB Protocol PDF Memory Recall (Memory)
Fillable Online IRB Minimal Risk Protocol Template Fax Email Print
Webinars · Courses · All Content
New Protocol Templates The Irb Office Has Developed Three New Protocol Templates For Use By The Northwestern University Research Community To Describe Human.
Related Post: